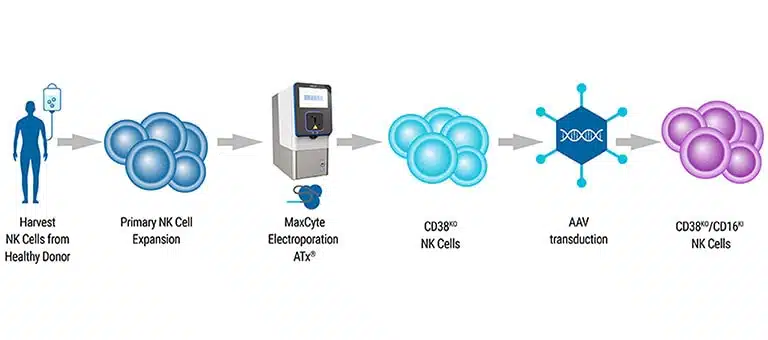
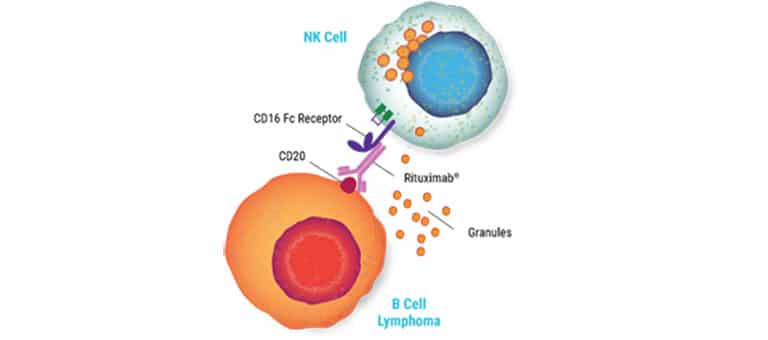
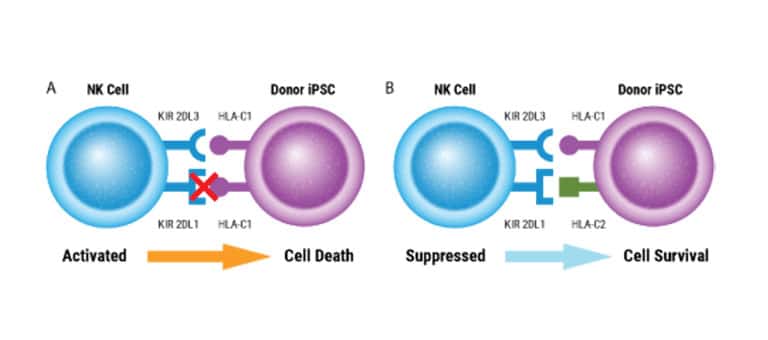
CAR NK Cell Manufacturing
Unlock the promise of NK cell therapy with highly efficient delivery of CAR constructs to NK cells using MaxCyte® ExPERTTM instruments.
Our gentle electroporation ensures high yields of viable, precisely engineered NK cells, for rapid, efficient and clinically scalable CAR NK cell manufacturing.
Introduction
NK Cell therapy is an exciting frontier in immunotherapy, that harnesses the potential of natural killer (NK) cells to target and eradicate cancer.
NK cells are isolated and expanded from peripheral blood or cord blood sources. They are then genetically modified to express Chimeric Antigen Receptors (CARs) on their surface. The introduction of CARs can be achieved through gene transfer techniques, including viral vectors or non-viral methods.
Following genetic modification, CAR NK cells undergo a phase of expansion and growth to obtain sufficient cell numbers for therapy.
Enable efficient, stable CAR expression
As with many primary cells, NK cells have proven challenging to engineer. Our electroporation technology enables highly efficient delivery of mRNA and is gentle on cells, resulting in increased high-affinity CD16 receptor expression. Partner with us to make your engineering easier.
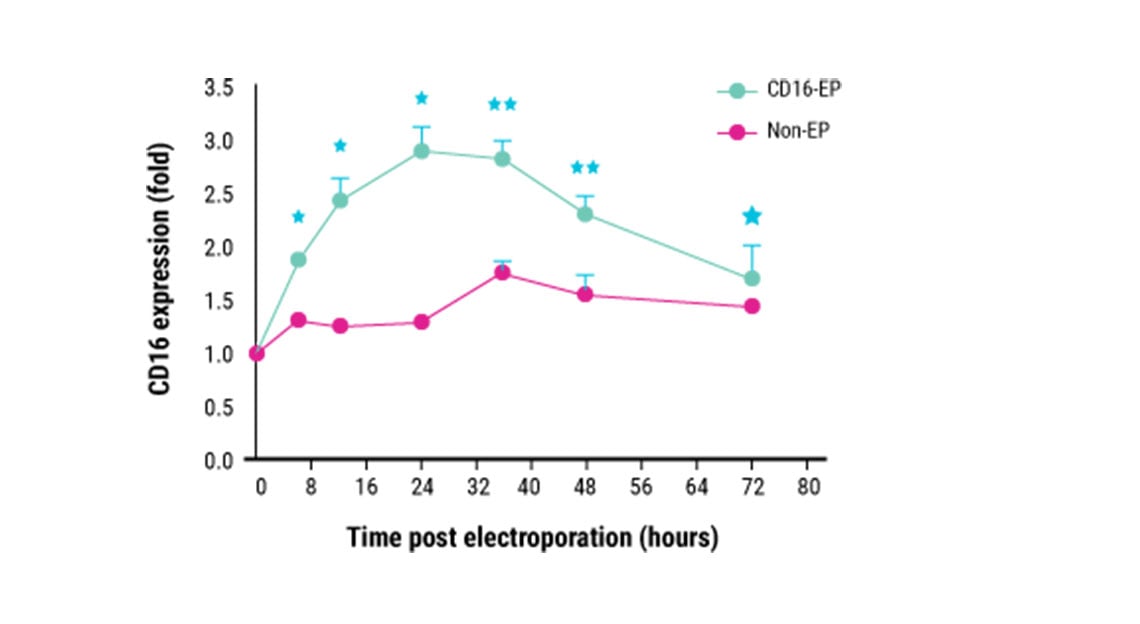
Kinetics of CD16 expression in ex vivo expanded NK cells.
This content was adapted from Mattias Carlsten et al. Front. Immunol. (2016) under the Creative Commons license Attribution 4.0 International (CC BY 4.0).
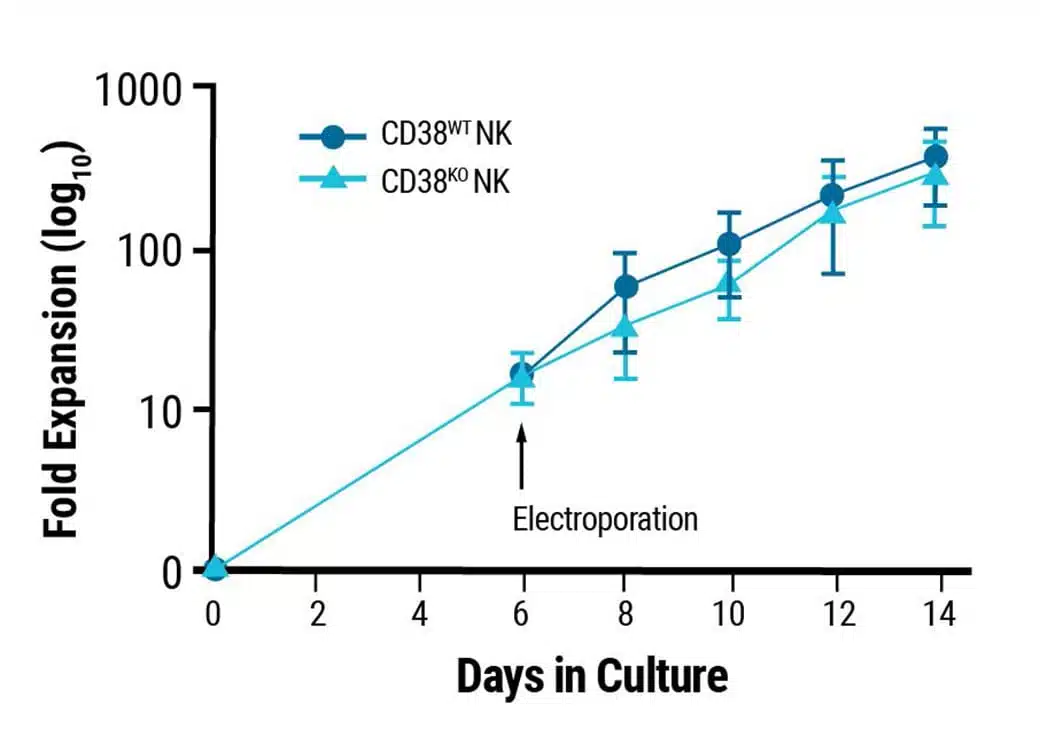
Proliferative characteristics of engineered NK cells remain unchanged.
This content was adapted from Joseph Clara et al. J Immunother Cancer. (2022) under the Creative Commons license Attribution 4.0 International (CC BY 4.0).
Ensure functional and proliferative edited cells
Disruption of healthy function is always a concern when engineering cells. At MaxCyte, we have spent 20 years optimizing electroporation. As a result, we have a wide range of cell-type specific protocols designed to protect the functional and proliferative capacity of your cells. Collaborate with us for electroporation that is simple, gentle and efficient.
Perform sophisticated multiplexed engineering
Intricate engineering strategies are being developed to boost CAR NK cell therapy efficiency and potency. MaxCyte empowers researchers with a highly efficient, easily adaptable, scalable solution for multiplexed genetic engineering. We're excited to collaborate to create a streamlined workflow for developing and manufacturing your immunotherapies.
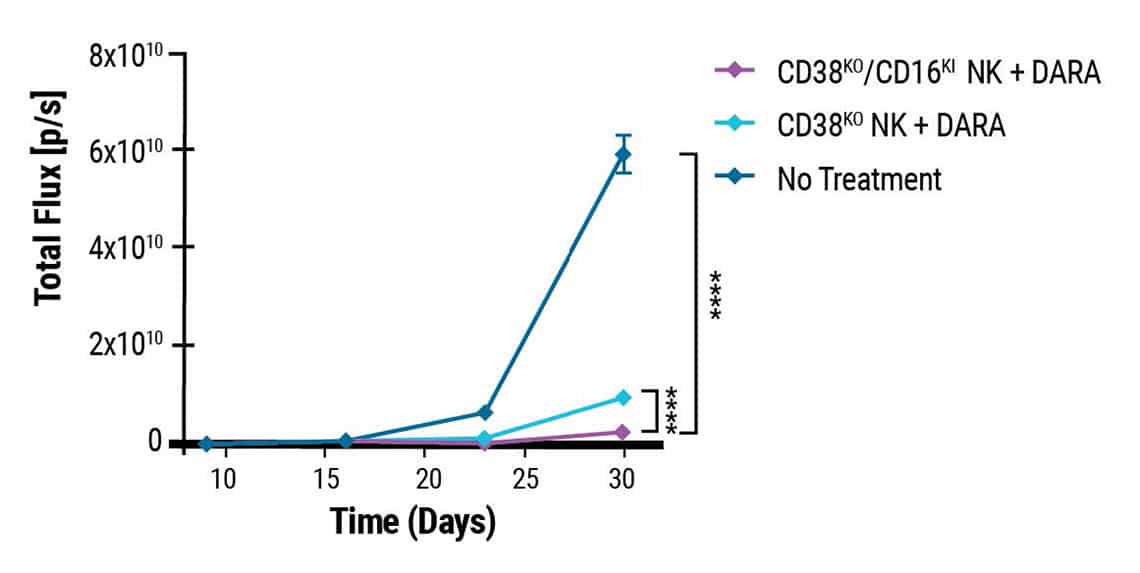
Double-edited NK cells show higher anti-myeloma activity in xenograft mice.
This content was adapted from Joseph Clara et al. J Immunother Cancer. (2022) under the Creative Commons license Attribution 4.0 International (CC BY 4.0).
Research Applications
Resources
ExPERT GTx™ Electroporation System
The ExPERT GTxTM is the only non-viral transfection system enabling a commercially approved cell therapy. Clinically-proven in over 45 clinical trials across various indications and gene editing tools, our ExPERT GTxTM system and on-demand scientific support helps accelerating development while mitigating risks.
Benefit from our proven track record of reducing preclinical development by multiple months with our scientific knowhow that extends beyond the electroporation step. Embrace continuous scalability with the only platform offering transition from 15uL to 100mL on a single instrument.


Reagents and Processing Assemblies
MaxCyte's consumable products provide users with a variety of options for project scale and throughput from discovery through cGMP manufacturing using a single platform. Our range of Processing Assemblies allows users to transfect a variety of cell sample volumes to meet specific application needs. MaxCyte's Electroporation Buffer is animal-derived component free and safe for all cell types ensuring consistent, high-performance transfection.
Ready to learn more about our technology?
Find out how we can help you reach your CAR NK cell manufacturing and research goals.